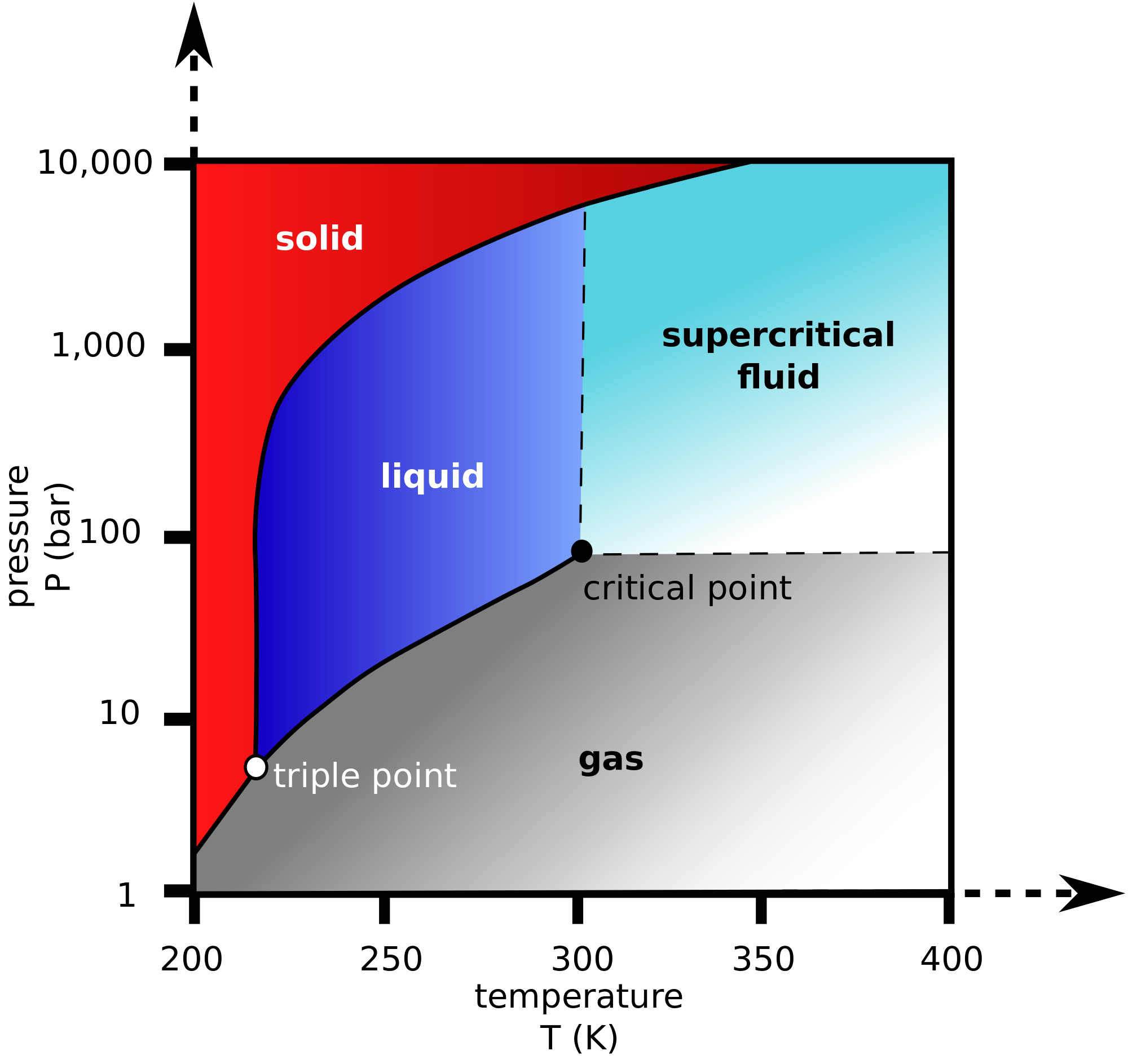
Nitrous Oxide Vapor Pressure Vs Temperature . what pressure does liquid nitrous oxide need at −45 °c to remain liquid? As the gaseous nitrous oxide escapes, more.
from socratic.org
As the gaseous nitrous oxide escapes, more. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =.the pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi.
What is the relation between critical temperature and boiling point or
Nitrous Oxide Vapor Pressure Vs Temperature Latent heat of vaporization (at boiling point) 374.286kj/kg. As the gaseous nitrous oxide escapes, more. the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale. what pressure does liquid nitrous oxide need at −45 °c to remain liquid?
From vacaero.com
Evaporation Nitrous Oxide Vapor Pressure Vs Temperature the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale. Latent heat of vaporization (at boiling point) 374.286kj/kg. As the gaseous nitrous oxide escapes, more. what pressure does liquid nitrous oxide need at −45 °c to remain liquid?the pressure of nitrous oxide vapor floating above the liquid nitrous oxide is. Nitrous Oxide Vapor Pressure Vs Temperature.
From socratic.org
What is the relation between critical temperature and boiling point or Nitrous Oxide Vapor Pressure Vs Temperature what pressure does liquid nitrous oxide need at −45 °c to remain liquid? How can i calculate its pressure. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. As the gaseous nitrous oxide escapes, more. Latent heat of. Nitrous Oxide Vapor Pressure Vs Temperature.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Nitrous Oxide Vapor Pressure Vs Temperaturethe pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. what pressure does liquid nitrous oxide need at −45 °c to remain liquid? the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale. As the gaseous nitrous oxide escapes, more. where p p is the. Nitrous Oxide Vapor Pressure Vs Temperature.
From wisc.pb.unizin.org
Vapor Pressure and Boiling Point Correlations (M10Q3) UWMadison Nitrous Oxide Vapor Pressure Vs Temperature where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =.the pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. what pressure does liquid nitrous oxide need at −45 °c to remain liquid?. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.researchgate.net
Vapor pressure versus temperature for the above oxides, indicating the Nitrous Oxide Vapor Pressure Vs Temperature what pressure does liquid nitrous oxide need at −45 °c to remain liquid? where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. As the gaseous nitrous oxide escapes, more. the vapour pressure of nitrous oxide has been. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.powerstream.com
Vapor pressures of the Chemical Elements, vapor pressure of metals and Nitrous Oxide Vapor Pressure Vs Temperature As the gaseous nitrous oxide escapes, more. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale.the pressure of nitrous oxide. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.sexiezpix.com
Vapor Pressure Chart For Water SexiezPixPorn Nitrous Oxide Vapor Pressure Vs Temperature where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =.the pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. How can i calculate its pressure. what pressure does liquid nitrous oxide need. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.pharmacology2000.com
Anesthesia Vaporization Nitrous Oxide Vapor Pressure Vs Temperature what pressure does liquid nitrous oxide need at −45 °c to remain liquid? where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. How can i calculate its pressure. As the gaseous nitrous oxide escapes, more. Latent heat of. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.microfinanceindia.org
vapor pressure of water table Nitrous Oxide Vapor Pressure Vs Temperaturethe pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. As the gaseous nitrous oxide escapes, more. the vapour pressure of nitrous oxide. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.speedwaymotors.com
NOS 14730BNOS Nitrous Bottle, 5 Pound, with Gauge, Black Nitrous Oxide Vapor Pressure Vs Temperature As the gaseous nitrous oxide escapes, more. what pressure does liquid nitrous oxide need at −45 °c to remain liquid? How can i calculate its pressure.the pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. Latent heat of vaporization (at boiling point) 374.286kj/kg. Nitrous Oxide Vapor Pressure Vs Temperature.
From digital.library.unt.edu
Vapor pressures of concentrated nitric acid solutions in the Nitrous Oxide Vapor Pressure Vs Temperature Latent heat of vaporization (at boiling point) 374.286kj/kg. the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale. As the gaseous nitrous oxide escapes, more.the pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. where p p is the pressure you want to solve for,. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.researchgate.net
Specific Heat variation of nitrous oxide w.r.t. temperature in a Nitrous Oxide Vapor Pressure Vs Temperature As the gaseous nitrous oxide escapes, more. what pressure does liquid nitrous oxide need at −45 °c to remain liquid? How can i calculate its pressure. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. Latent heat of. Nitrous Oxide Vapor Pressure Vs Temperature.
From almerja.com
Properties of Liquids Nitrous Oxide Vapor Pressure Vs Temperature Latent heat of vaporization (at boiling point) 374.286kj/kg. what pressure does liquid nitrous oxide need at −45 °c to remain liquid? As the gaseous nitrous oxide escapes, more. How can i calculate its pressure. the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.bartleby.com
Vapor pressure curves for CS 2 (carbon disulfide) and CH 3 NO 2 Nitrous Oxide Vapor Pressure Vs Temperature where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. As the gaseous nitrous oxide escapes, more. what pressure does liquid nitrous oxide need at −45 °c to remain liquid? Latent heat of vaporization (at boiling point) 374.286kj/kg. How. Nitrous Oxide Vapor Pressure Vs Temperature.
From present5.com
Water vapor Nitrous oxide Aerosols Structure of Nitrous Oxide Vapor Pressure Vs Temperature what pressure does liquid nitrous oxide need at −45 °c to remain liquid? the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.researchgate.net
Saturated liquid density of stored nitrous oxide in wide range of Nitrous Oxide Vapor Pressure Vs Temperature the vapour pressure of nitrous oxide has been rigorously measured since the first international temperature scale. what pressure does liquid nitrous oxide need at −45 °c to remain liquid? where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2. Nitrous Oxide Vapor Pressure Vs Temperature.
From rocketprops.readthedocs.io
N2O — RocketProps 0.1.7 documentation Nitrous Oxide Vapor Pressure Vs Temperature what pressure does liquid nitrous oxide need at −45 °c to remain liquid?the pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =.. Nitrous Oxide Vapor Pressure Vs Temperature.
From www.researchgate.net
P − ρ diagram for nitrous oxide injection. Isothermal line at 293K. The Nitrous Oxide Vapor Pressure Vs Temperaturethe pressure of nitrous oxide vapor floating above the liquid nitrous oxide is 750 psi. How can i calculate its pressure. where p p is the pressure you want to solve for, pc = 7251 p c = 7251 kpa, b1 = −6.71893 b 1 = − 6.71893, b2 =. the vapour pressure of nitrous oxide has. Nitrous Oxide Vapor Pressure Vs Temperature.